| Formula |
CAS |
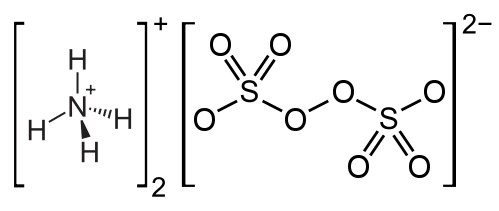
| (NH4)2S2O8 |
7727-54-0 |
BackgroundAmmonium persulfate is (NH4)2S2O8 is a strong
oxidizing agent and bleacher in hair bleaches. It is very soluble in water;
the dissolution of the salt in water is endothermic. It is a radical initiator.
It is used to etch copper on printed circuit boards as an alternative to ferric
chloride solution. It is also used along with tetramethylethylenediamine to
catalyze the polymerization of acrylamide in making a polyacrylamide gel. Ammonium
persulfate is the main component of Nochromix. On dissolving in sulfuric acid,
it is used to clean laboratory glassware as a metal-free alternative to chromic
acid baths. It is also a standard ingredient in western blot gels and hair bleach.
SynonymsAmmonium persulfate Ammonium peroxydisulfate
UsesCleaning laboratory glassware
Western blot gels
Oxidizer and bleacher in hair bleaches
Decolorizing and deodorizing oils
Electorplating
Manufacturing aniline dyes
Oxidizer for copper
Making
soluble starch
Analytical chemistry
Depolarizer in electric batteries
Reducer and retarder in photography
Cross-Reactions
Unusual ReactionsAirborne contact dermatitis
Contact
urticaria
Airborne dust may be irritating to eye, nose, throat, lung and
skin upon contact. Exposure to high levels of dust may cause difficulty in breathing.
Back to list of contact allergens
Referenties
| 1. |
Maibach HI, Johnson HL. Contact urticaria
syndrome. Contact urticaria to diethyltoluamide (immediate-type
hypersensitivity). Arch Dermatol 1975;111(6):726-730. |
| 2. |
Fisher AA, Dooms-Goossens A. Persulfate hair
bleach reactions. Cutaneous and respiratory manifestations. Arch
Dermatol 1976;112(10):1407-1409. |
| 3. |
Winton GB, Lewis CW. Contact urticaria. Int
J Dermatol 1982;21(10):573-578. |
| 4. |
van der Walle HB, Brunsveld VM. Dermatitis
in hairdressers. (I). The experience of the past 4 years. Contact
Dermatitis 1994;30(4):217-221. |
Author(s):Allergology: background information on allergens.
